Standard Enthalpy of Combustion
I highlighted 1 mole of water because thats what I used to solve the problem. The superscript degree symbol indicates that substances are in their standard states.

Enthalpy Of Combustion Easy Science Learn Physics Chemical Energy Thermodynamics
In this equation dW is equal to dW pdV and is known as the boundary work.

. The heating value or energy value or calorific value of a substance usually a fuel or food see food energy is the amount of heat released during the combustion of a specified amount of it. Brayton cycle or Rankine cycle. The calorific value is the total energy released as heat when a substance undergoes complete combustion with oxygen under standard conditionsThe chemical reaction is typically a.
Boundary work occurs because the mass of the substance. The first law of thermodynamics in terms of enthalpy show us why engineers use the enthalpy in thermodynamic cycles eg. DU dQ dW.
ΔH ΔG S Definitions of standard states. The classical form of the law is the following equation. The term standard state is used to describe a reference state for substances and is a help in thermodynamical calculations as enthalpy entropy and Gibbs free energy calculations.
According to the definition of enthalpy of neutralization chem libretexts the standard enthalpy change of neutralization is the enthalpy change when solutions of an acid and an alkali react together under standard conditions to produce 1 mole of water. For a gas the standard state is as a pure gaseous.

Finding Change In Enthalpy Of A Reaction From Change In Formation Values Of Reactants And Products By Applying Hess S Law Physique Chimie Chimie Scientifique
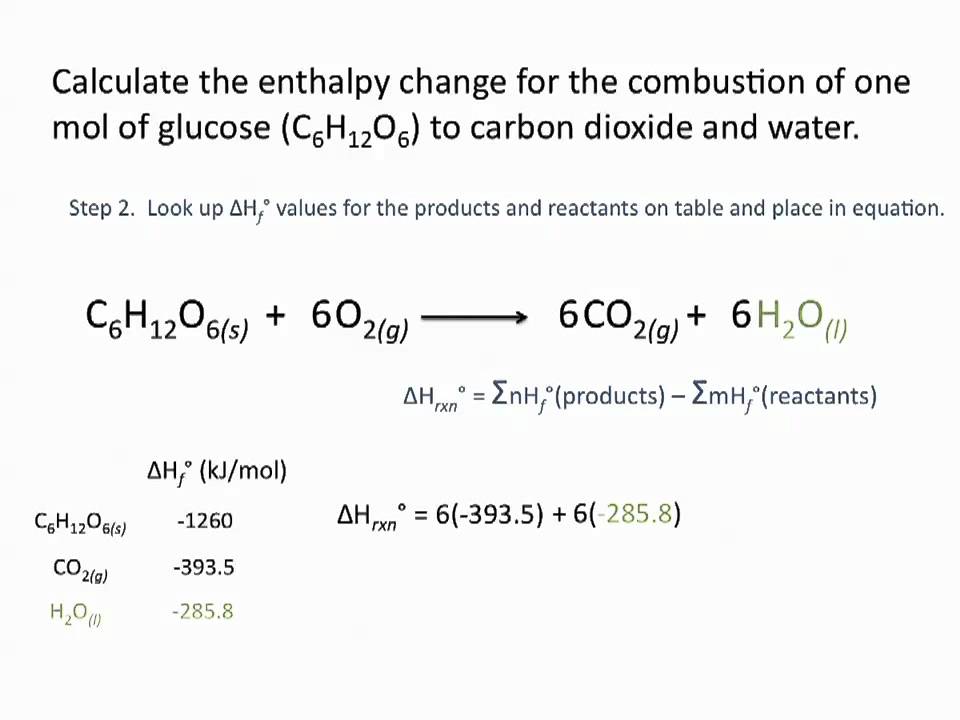
Enthalpies Of Formation Chemsitry Tutorial Science Chemistry Chemistry Tutorial

Total 2 Average 5 X2f 5 What Is The Heat Of Combustion What Is The Definition Of Enthalpy Chemistry Lessons Chemistry Classroom How To Study Physics

What Is The Heat Of Combustion A Plus Topper Https Www Aplustopper Com Enthalpy Heat Combustion Heat Of Combustio Heat Heat Energy Exothermic Reaction

Comments
Post a Comment